Answer : The partial pressure of
 is, 12.34 atm
is, 12.34 atm
Explanation :
For the given chemical reaction:
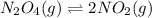
The expression of
 for above reaction follows:
for above reaction follows:
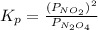 ........(1)
........(1)
The equilibrium reaction is:
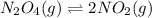
Initial x 0
At eqm (x-y) 2y
Putting values in expression 1, we get:
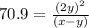 ..............(2)
..............(2)
As we are given that, 25.8 % of
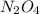 remains at equilibrium. That means,
remains at equilibrium. That means,
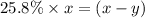
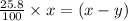
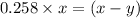
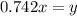 ..............(3)
..............(3)
Now put equation 3 in 2, we get the value of 'x'.
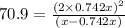
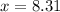
Now put the value of 'x' in equation 3, we get:
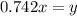
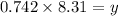
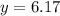
Now we have to calculate the new partial pressure of
 at equilibrium.
at equilibrium.
Partial pressure of
 = (2y) = (2\times 6.17) = 12.34 atm
= (2y) = (2\times 6.17) = 12.34 atm
Hence, the partial pressure of
 is, 12.34 atm
is, 12.34 atm