Step-by-step explanation:
A covalent bond is defined as the occurrence of a bond due to the sharing of electrons between the combining atoms.
Atomic number of hydrogen atom is 1 and its electronic configuration is
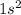 . So, in order to complete its octet it needs to gain or mutually shares one electron.
. So, in order to complete its octet it needs to gain or mutually shares one electron.
A covalent bond is generally formed between non-metal atoms.
Thus, we can conclude that hydrogen has only one electron that will be involved in the formation of a covalent bond.