Answer:
c=326.5177 J/kg.K
Specific heat is c=326.5177 J/kg.K
Step-by-step explanation:
In order ti find the specific heat, we will proceed as follow:
Formula we are going to use is:

Where:
Q is the heat energy added
m is the mass of sample
c is the specific heat
 is the temperature Rise.
is the temperature Rise.
First we will find the mass:
Weight=m*g (g is gravitational acceleration=9.8 m/s^2)
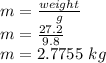
Rearranging above formula:
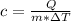
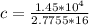
c=326.5177 J/kg.K
Specific heat is c=326.5177 J/kg.K