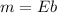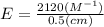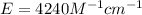Answer:
The extinction coefficient is 4240 M⁻¹cm⁻¹
Step-by-step explanation:
The Beer-Lambert law for the absorbance of a monochromatic light by a molecule is given by the following mathematical expression:
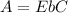 ----------------------(1)
----------------------(1)
Here, E is the extinction coefficient, b is the length of light pathway through the solution and C is the concentration in mol/L. This is a linear equation and fits well with its formula
y = mx + C-------------------(2)
Here, m is the slope and C is the y intercept. y and x are the two variables. Comparing equation 1 and 2, Absorbance (A) and molar concentration (C) are the two variables, while the product of extinction coefficient (E) and path length (b) makes the slope of the line. The y-intercept in the given equation is due to the solvent interference.
From this information, we can determine the extinction coefficient by the slope of the line with the following formula.