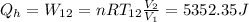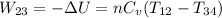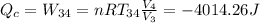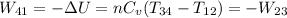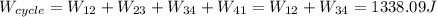Answer:
The work performed during the cycle W is 1338.09J. The heat absorbed from the hot source Qh is 5352.35J. The heat released to the cold source Qc is 4014.26J.
Step-by-step explanation:
If we considered the cycle done between four points, the initial point 1 to be the point of the initial volume (V₁=1L) and the next to be the final volume on that isothermal transformation (V₂=5L with T₁₂=400K).
Using the adiabatic relationship between Volume and temperature:
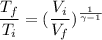
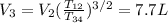
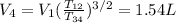
For the cycle: