Step-by-step explanation:
It is known that more linearly the atoms are arranged in a carbon chain more will be its melting point. This is because then more surface area has been occupied by the molecule and in order to break all the bonds, heat energy has to reach to the entire surface area.
As a result, melting point of the compound will be high. On the other hand, more is the branching present in a carbon chain less will be the surface area occupied by it. Hence, smaller will be its melting point.
Therefore, in the given structures of (
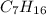 ) as the branching increases there occurs a decrease in melting point.
) as the branching increases there occurs a decrease in melting point.