Answer: The formula unit equation is written below.
Step-by-step explanation:
Formula unit equation is defined as the balanced chemical equation that includes physical state of matter of all the compounds.
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on product side.
When ammonium fluoride reacts with magnesium chloride, it leads to the formation of ammonium chloride and a solid precipitate of magnesium fluoride.
The formula unit equation for the reaction of ammonium fluoride and magnesium chloride follows:
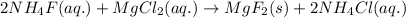
This is an example of double displacement reaction.
Hence, the formula unit equation is written above.