Answer:
The heat is 115478.4 J.
Step-by-step explanation:
Given that,
Mass of water = 0.400 kg
Power = 200 W
Suppose, we determine how much heat must be added to the water to raise its temperature from 20.0°C to 89.0°C?
We need to calculate the heat
Using formula of heat
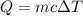
Where, m = mass of water
c = specific heat
Put the value into the formula
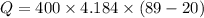
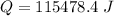
Hence, The heat is 115478.4 J.