Answer:
[H⁺] = 16.6 (±1.9) x10⁻⁴ M
Step-by-step explanation:
The pH is defined as:
So we can calculate [H⁺]:
- [H⁺] =
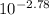
[H⁺] = 1.66x10⁻³ M
The relative uncertainty in [H⁺] is
- uR/R = 2.303 x AbsoluteUncertainty
- uR/R = 2.303 * 0.05 = 0.115
Thus the uncertainty in the concentration is:
1.66x10⁻³ M * 0.115 = 1.91x10⁻⁴ M