Answer: Option (1) is the correct answer.
Step-by-step explanation:
As per Le Chatelier's principle, any disturbance caused in an equilibrium reaction will tend to shift the equilibrium in a direction away from the disturbance.
For example,
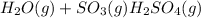
As this given reaction is endothermic in the forward direction and exothermic in the backward direction. Thus, in order to shift the reaction on left side we need to decrease the temperature.
Also, the number of moles are more on reactant side as compared to product side. So, when we decrease the number of moles on reactant side then the equilibrium will shift on left side.
Therefore, we can conclude that for the given reaction decreasing the pressure of the system and lowering the temperature of the system would shift the reaction to the left.