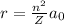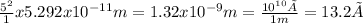Answer: 13.2 Å
Explanation:
Bohr's model of atom stated that the electrons revolves around the nucleus in those orbits which have fixed energy and do not lose it. The energy at infinite distance is taken to be zero and as it approaches the atom it starts becoming more negative. And, n is the principal quantum number which indicates the x shell where the electron is found.
The energy is quantized, which means it is allowed only in certain values. And the separation of energy depends on the mass and motion of the electron. Each energy level has a specific value (
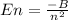 ), where n is 1, 2, 3, 4, 5.... and B is a constant which has a value of 2.179 x
), where n is 1, 2, 3, 4, 5.... and B is a constant which has a value of 2.179 x
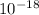 Joules.
Joules.
The energy, stated by this question, is -8.72 x
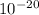
Calculate n:
Z is the atomic number or proton number, which indicates the chemical element. Hydrogen has an atomic number of 1.
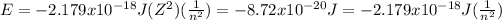
n =
 = 4.47 = 5
= 4.47 = 5
Then, calculate the ratio using the formula