Answer:
The mass of the bar = 199.87 grams
Step-by-step explanation:
Given:
Density of a brass bar = 9.87
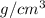
Volume of bar = 20.25
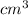
To find the mass of the brass bar.
Solution:
Density of a body is defined as mass of the body per unit volume of the body.
Thus, we have:
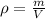
where:
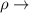 density of the body
density of the body
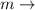 mass of the body
mass of the body
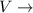 Volume of the body
Volume of the body
Plugging in the given values.
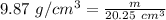
Solving for

Multiplying both sides by 20.25
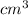 .
.
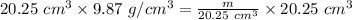
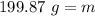
∴
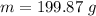
Thus, the mass of the bar = 199.87 grams