Answer:
![[Fe^(+3)]=0.700 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/mv4oaoswxpjug6xs2llh872wbu55icjmde.png)
![[NO_(3)^(-)]=2.10 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/6o0h2vxt6ykpodrlz28rjy891ue2lxwrou.png)
Step-by-step explanation:
Here, a solution of Fe(NO₃)₃ is diluted, as the total volume of the solution has increased. The formula for dilution of the compound is mathematically expressed as:
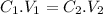
Here, C and V are the concentration and volume respectively. The numbers at the subscript denote the initial and final values. The concentration of Fe(NO₃)₃ is 1.75 M. As ferric nitrate dissociates completely in water, the initial concentration of ferric is also 1.75 M.
Solving for [Fe],
![[Fe^(+3)]=(C_(1).V_(1))/(V_(2) )](https://img.qammunity.org/2021/formulas/chemistry/high-school/5pus8hv3nvo3l5ovcdqmcuhpkrjpl3hhqx.png)
![[Fe^(+3)]=((1.75).(30.0))/(45.0+30.0 )](https://img.qammunity.org/2021/formulas/chemistry/high-school/car8jtj2vaaqgb3mi7dpg0ejrffz92hb40.png)
![[Fe^(+3)]=0.700 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/mv4oaoswxpjug6xs2llh872wbu55icjmde.png)
For [NO₃⁻],
There are three moles of nitrate is 1 mole of Fe(NO₃)₃. This means that the initial concentration of nitrate ions will be three times the concentration of ferric nitrate i.e., it will be 5.25 M.
![[NO_(3)^(-)]=(C_(1).V_(1))/(V_(2) )](https://img.qammunity.org/2021/formulas/chemistry/high-school/ojppkqzw177g3vpzejbc1e9luj7f09aelc.png)
![[NO_(3)^(-)]=((5.25)(30.0))/(30.0+45.0 )](https://img.qammunity.org/2021/formulas/chemistry/high-school/ujk3763c03hwbl5pirk5dqregov1dz3ww6.png)
![[NO_(3)^(-)]=2.10 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/6o0h2vxt6ykpodrlz28rjy891ue2lxwrou.png)