The question is incomplete, here is the complete question:
Sulfuric acid is essential to dozens of important industries from steel making to plastics and pharmaceuticals. More sulfuric acid is made than any other industrial chemical, and world production exceeds
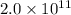 per year.
per year.
The first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. Suppose an engineer studying this reaction introduces 4.4 kg of solid sulfur and 6.90 atm of oxygen gas at 950°C into an evacuated 50.0 L tank. The engineer believes
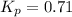 for the reaction at this temperature.
for the reaction at this temperature.
Calculate the mass of solid sulfur he expects to be consumed when the reaction reaches equilibrium. Round your answer to 2 significant digits.
Answer: The mass of sulfur that is expected to be consumed is 0.046 kg
Step-by-step explanation:
We are given:
Initial partial pressure of oxygen gas = 6.90 atm
The chemical equation for the formation of sulfur dioxide follows:
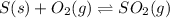
Initial: 6.90
At eqllm: 6.90-x x
The expression of
 for above equation follows:
for above equation follows:
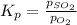
We are given:
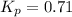
Putting values in above equation, we get:

So, equilibrium partial pressure of sulfur dioxide = x = 2.9 atm
To calculate the number of moles, we use the equation given by ideal gas, which follows:
PV = nRT
where,
P = pressure of sulfur dioxide gas = 2.9 atm
V = volume of the container = 50.0 L
n = number of moles of sulfur dioxide gas = ?
R =
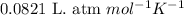
T = temperature of the container =
![950^oC=[950+273]K=1223K](https://img.qammunity.org/2021/formulas/chemistry/college/ji6gm2d0dt4c2tt4h53kqf6f0pbbsmoptr.png)
Putting values in above equation, we get:
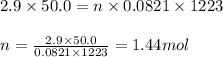
Moles of sulfur dioxide = 1.44 moles
By Stoichiometry of the reaction:
1 mole of sulfur dioxide is produced from 1 mole of sulfur
So, 1.44 moles of sulfur dioxide will be produced from
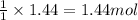 of sulfur
of sulfur
To calculate the mass of a substance, we use the equation:

Moles of sulfur = 1.44 moles
Molar mass of sulfur = 32 g/mol
Putting values in above equation, we get:
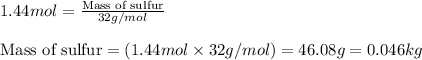
Hence, the mass of sulfur that is expected to be consumed is 0.046 kg