This is an incomplete question, here is a complete question.
Determine whether each of the following electron configurations is an inert gas, a halogen, an alkali metal, an alkaline earth metal, or a transition metal. Justify your choices.
(a)
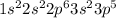
(b)
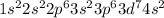
(c)
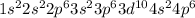
(d)
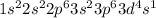
Answer :
(a)
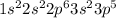 → Halogen
→ Halogen
(b)
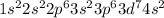 → Transition metal
→ Transition metal
(c)
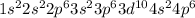 → Transition metal
→ Transition metal
(d)
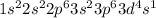 → Transition metal
→ Transition metal
Explanation :
Inert gas : These are the gases which lie in group 18.
Their general electronic configuration is:
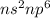 where n is the outermost shell.
where n is the outermost shell.
Halogen : These are the elements which lie in group 17.
Their general electronic configuration is:
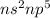 where n is the outermost shell.
where n is the outermost shell.
An alkali metal : These are the elements which lie in group 1.
Their general electronic configuration is:
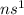 where n is the outermost shell.
where n is the outermost shell.
An alkaline earth metal : These are the elements which lie in group 2.
Their general electronic configuration is:
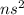 where n is the outermost shell.
where n is the outermost shell.
Transition elements : They are the elements which lie between 's' and 'p' block elements. These are the elements which lie in group 3 to 12. The valence electrons of these elements enter d-orbital.
Their general electronic configuration is:
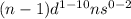 where n is the outermost shell.
where n is the outermost shell.
(a)
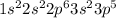
The element having this electronic configuration belongs to the halogen family.
(b)
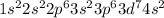
The element having this electronic configuration belongs to the transition family.
(c)
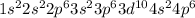
The element having this electronic configuration belongs to the transition family.
(d)
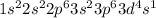
The element having this electronic configuration belongs to the transition family.