This is an incomplete question. The complete question is :
Calculate the change in internal energy (ΔE) for a system that is giving off 25.000 kJ of heat and is changing from 18.00 L to 15.00 L in volume at 1.50 atm pressure.
Answer: The change in internal energy for a system is -24544 Joules
Step-by-step explanation:
According to first law of thermodynamics:
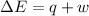
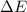 =Change in internal energy
=Change in internal energy
q = heat absorbed or released
w = work done or by the system
w = work done by the system=
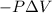 {Work done on the system as the final volume is lesser than initial volume and is positive}
{Work done on the system as the final volume is lesser than initial volume and is positive}
w =
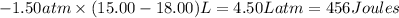 {1Latm=101.3J}
{1Latm=101.3J}
q = -25.000 kJ =
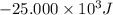 {Heat released by the system is negative}
{Heat released by the system is negative}
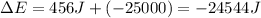
Thus change in internal energy for a system is -24544 Joules