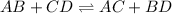Answer:
The correct answer is :
b. Single displacement reaction
Step-by-step explanation:
The single displacement reaction is the one in which the more reactive element substitute other element in a compound generating a new chemical compound.
It follows this kind of reactions :
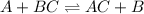
Now look at the reaction given;
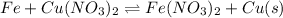
Here the more reactive element (Fe) displaces the less reactive element [Cu]from the compound[Cu(NO3)2].
Hence Fe substitute Cu from Cu(NO3)2 and form Fe(NO3)2
It is not a double - displacement reaction . Where the elements interchange themselves to form the compound.