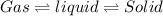Answer:
The state of the element or compound decided by two forces mainly:
The force of the attraction
The Kinetic(thermal) energy between the molecules of the element.
Step-by-step explanation:
Kinetic Energy depends upon the temperature , and on increasing the temperature the kinetic energy also increases.
Force of attraction between the particles is increased by applying pressure to the substance.
When the substance is in solid state then , the force of attraction between the particles is much more then the thermal energy of the particles.
The thermal energy or kinetic energy reduces the force of attraction between the particles.Hence if the kinetic energy is increased then the substances goes from solid to liquid state.
Thus on, increasing the temperature the substance changes the state from solid to liquid
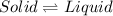
If you increase further , the kinetic energy then the substance goes from liquid to gaseous state again.
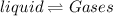
Role of Kinetic energy : The kinetic energy separate the particles further to large distance.Hence they become far apart result in change in state.
Affect of increasing pressure produce the reverse products.