 number of particles are present in 2.75 moles of Aluminium fluoride
number of particles are present in 2.75 moles of Aluminium fluoride
Explanation:
- The number of particles in 1 mole of substance is
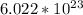 which is called the Avogadro’s number.
which is called the Avogadro’s number. - It is is a number which represents a atomic scale substance to a understandable human scale form.
- Here, 1 mole of aluminium fluoride contains
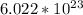 number of particles. Hence, to find the number of particles in 2.75 moles, you just multiply it with Avogadro's number.
number of particles. Hence, to find the number of particles in 2.75 moles, you just multiply it with Avogadro's number.
So, the number of particles in 2.75 moles of aluminium fluoride is
