Answer:
3500
Step-by-step explanation:
The equilibrium constant of the reaction which has to be calculated is:-
2 NO2 (g) ⇋ N2(g) + 2 O2(g)
The given chemical equation follows:
1/2 N2 (g) + 1/2 O2 (g) ⇋ NO (g)
The equilibrium constant for the above equation is 0.004.
We need to calculate the equilibrium constant for the reverse equation of above chemical equation and also double of the above chemical equation, which is:
2NO (g) ⇋ N2 (g) + O2 (g)
The equilibrium constant for the reverse double reaction will be the reciprocal of the initial reaction.
If the equation is multiplied by a factor of '2', the equilibrium constant of the reverse reaction will be the square of the equilibrium constant of initial reaction.
The value of equilibrium constant for reverse reaction is:
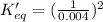 = 62500
= 62500
The given chemical equation follows:
2NO2 (g) ⇋ 2NO (g) + O2 (g)
The equilibrium constant for the above equation is 0.056.
Thus, adding the both reaction gives the original reaction. So, equilibrium constant is:- 62500 x 0.056 = 3500