The question is incomplete, here is the complete question:
Suppose the reaction is:
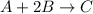
If 6 moles of A and 2 moles of B are reacted, what is the maximum number of moles of C that can be formed
Answer: The maximum amount of C produced will be 1 mole
Step-by-step explanation:
We are given:
Moles of A = 6 moles
Moles of B = 2 moles
For the given chemical reaction:
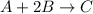
By Stoichiometry of the reaction:
2 moles of B reacts with 1 mole of A
So, 2 moles of B will react with =
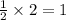 mole of A
mole of A
As, As, given amount of A is more than the required amount. So, it is considered as an excess reagent.
Thus, B is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
2 moles of B produces 1 mole of C
So, 2 moles of given B will produce
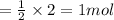 of C
of C
Hence, the maximum amount of C produced will be 1 mole