Answer:
a. The balanced chemical equation for the reaction
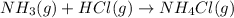
b. HCl is the limiting reagent.
c. 7.3295 grams of ammonium chloride could form from the reaction mixture in part (b).
d. 0.6715 grams of ammonia is left over in the reaction mixture in part b.
Step-by-step explanation:
a. The balanced chemical equation for the reaction
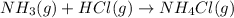
b.
Moles of ammonia =
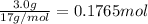
Moles of HCl =

According to reaction, 1 mole of HCl reacts with 1 mole of ammonia . Then 0.1370 moles of HCl will react with :
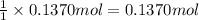 of ammonia
of ammonia
Hence, HCl is the limiting reagent.
c.
Since, HCl is a limiting reagent amount of ammonium chloride will depend upon moles of HCl.
According to reaction, 1 mole of HCl gives with 1 mole of ammonium chloride . Then 0.1370 moles of HCl will give :
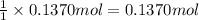 of ammonium chloride
of ammonium chloride
Mass of 0.1370 moles of ammonium chloride :
53.5 g/mol × 0.1370 mol = 7.3295 g
7.3295 grams of ammonium chloride could form from the reaction mixture in part (b).
d.
Moles of ammonia =
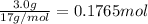
HCl is a limiting reagent and ammonia is an excessive reagent.
According to reaction, 1 mole of HCl reacts with 1 mole of ammonia . Then 0.1370 moles of HCl will react with :
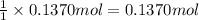 of ammonia
of ammonia
Moles of Ammonia reacted = 0.1370 mol
Moles of ammonia left unreacted = 0.1765 mol - 0.1370 mol = 0.0395 mol
Mass of 0.0395 moles of ammonia :
0.0395 mol × 17 g/mol = 0.6715 g
0.6715 grams of ammonia is left over in the reaction mixture in part b.