To solve this problem we will apply the concepts related to the equations of ideal gas, from there, we will define the proportion of these values between two states of matter. The ideal gas equation is defined as
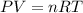
Here,
P = Pressure
V = Volume
n = Amount of mass
R = Gas ideal constant
T = Temperature
Between two states we have,
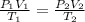
The pressure at each state is,
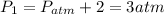
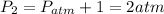
According to the statement,
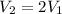
Then replacing,
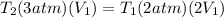
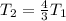
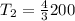
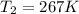
Therefore the correct answer is A.