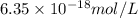Answer : The molar concentration is,
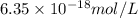
Explanation :
First we have to calculate the moles of protein.
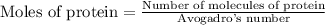
Number of molecules of protein = 2 molecules
Avogadro's number =
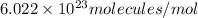
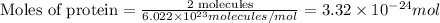
Now we have to calculate the radius.
Radius =
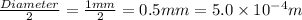
Conversion used : (1 mm = 0.001 m)
Now we have to calculate the volume.
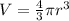
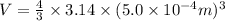
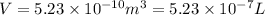
Conversion used : (1 m³ = 1000 L)
Now we have to calculate the molar concentration.
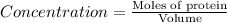
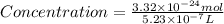
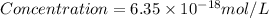
Thus, the molar concentration is,