Answer:
455.4 g (NH_4)2 SO_4
Step-by-step explanation:
Given:
no of moles of (NH_4)2 SO_4 , n=3.45 moles
To find:
The mass (w) of (NH_4)2 SO_4 in 3.45 moles (n)of it.
Molar mass (M) of (NH_4)2 SO_4 = 132 g
No of moles n is given by the formula;
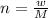
n is no. of moles
w is mass of the compound
M is the molar mass of the compound
Substituting the values in the formula,
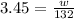
w=3.45 × 132=455.4g