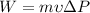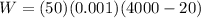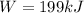To solve this problem we will start by differentiating the values in each of the states of matter. Subsequently through the thermodynamic tables we will look for the values related to the entropy, enthalpy and respective specific volumes. Through the relationship of Power defined as the product between mass and enthalpy and mass, specific volume and pressure, we will find the energetic values in the two states investigated. We will start defining the states
State 1
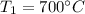
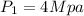
From steam table
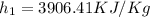
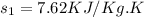
Now
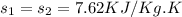 As 1-2 is isentropic
As 1-2 is isentropic
State 2
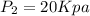
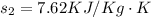
From steam table
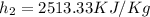
PART A) The power produced by turbine is the product between the mass and the enthalpy difference, then
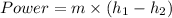
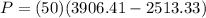
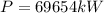
b) Pump Work
State 3
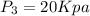
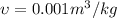
The Work done by the pump is