Answer : The mass percentage of barium in the compound is, 53.8 %
Explanation : Given,
Mass of barium compound = 441 mg
Mass of barium sulfate = 403 mg = 0.403 g (1 mg = 0.001 g)
The balanced chemical reaction will be:
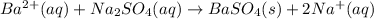
First we have to calculate the moles of
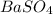
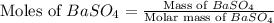
Molar mass of
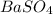 = 233.38 g/mole
= 233.38 g/mole
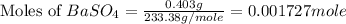
Now we have to calculate the moles of barium ion.
From the balanced chemical reaction, we conclude that
As, 1 mole of barium sulfate produced from 1 mole of barium ion
So, 0.001727 mole of barium sulfate produced from 0.001727 mole of barium ion
Now we have to calculate the mass of barium ion.
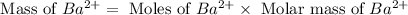
Molar mass of barium = 137.3 g/mol
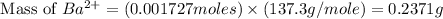
Now we convert the mass of barium ion from gram to mg.
Conversion used : (1 g = 1000 mg)
Mass of barium ion = 0.2371 g = 237.1 mg
Now we have to calculate the mass percentage of barium in the compound.
Mass percent of barium =
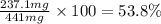
Thus, the mass percentage of barium in the compound is, 53.8 %