Answer:
(a). The weight of the system is 336.32 N.
(b). The molar volume is 16.6 m³/k mol.
The mass based volume is 0.145 m³/kg.
Step-by-step explanation:
Given that,
Weight of octane = 0.3 kmol
Volume = 5 m³
(a). Molecular mass of octane
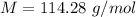
We need to calculate the mass of octane
Mass of 0.3 k mol of octane is
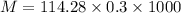
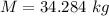
We need to calculate the weight of the system
Using formula of weight
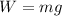
Put the value into the formula
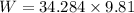
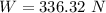
(b). We need to calculate the molar volume
Using formula of molar volume
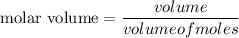
Put the value into the formula
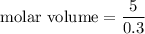
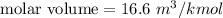
We need to calculate the mass based volume
Using formula of mass based volume
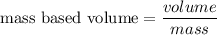
Put the value into the formula
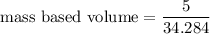
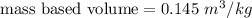
Hence, (a). The weight of the system is 336.32 N.
(b). The molar volume is 16.6 m³/k mol.
The mass based volume is 0.145 m³/kg.