Answer: No crystals of potassium sulfate will be seen at 0°C for the given amount.
Step-by-step explanation:
We are given:
Mass of potassium nitrate = 47.6 g
Mass of potassium sulfate = 8.4 g
Mass of water = 130. g
Solubility of potassium sulfate in water at 0°C = 7.4 g/100 g
This means that 7.4 grams of potassium sulfate is soluble in 100 grams of water
Applying unitary method:
In 100 grams of water, the amount of potassium sulfate dissolved is 7.4 grams
So, in 130 grams of water, the amount of potassium sulfate dissolved will be
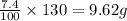
As, the soluble amount is greater than the given amount of potassium sulfate
This means that, all of potassium sulfate will be dissolved.
Hence, no crystals of potassium sulfate will be seen at 0°C for the given amount.