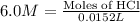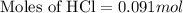This is an incomplete question, here is a complete question.
For each trial, calculate the number moles of 6.0 M HCl used in the reaction?
Trial 1 : Volume of HCl = 15.0ml
Trial 2 : Volume of HCl = 14.9ml
Trial 3 : Volume of HCl = 15.2ml
Answer :
The number moles of HCl for trial 1, 2 and 3 is, 0.090 mol, 0.089 mol and 0.091 mol
Explanation :
Molarity : It is defined as the number of moles of solute present in one liter of volume of solution.
Formula used :
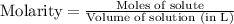
In this question, the solute is HCl.
Now we have to calculate the number of moles of HCl for trial 1.
Volume of HCl = 15.0 mL = 0.015 L
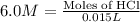
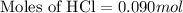
Now we have to calculate the number of moles of HCl for trial 2.
Volume of HCl = 14.9 mL = 0.0149 L
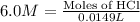
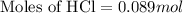
Now we have to calculate the number of moles of HCl for trial 3.
Volume of HCl = 15.2 mL = 0.0152 L