Answer: the calculation of the number of atoms conserved in the desired product rather than in waste.
Step-by-step explanation:
Atom economy gives how much desired product is obtained compared to amount of starting materials.
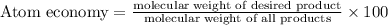
For example:
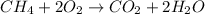
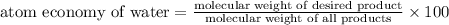
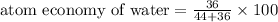
Thus atom economy for water is 45%