Answer:
The average atomic mass can be calculated from the exact atomic mass of each isotope by multiplying the mass each isotope by its relative abundance and then finding the sum of the result.
for example carbon has two major isotopes ;carbon 12 ,atomic mass with relative abundance of 98.9% and carbon 13 , atomic mass of with relative abundance 1.1%. The relative atomic mass is calculated below.
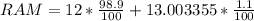
RAM=11.868 + 0.143036905
RAM=12.011036905amu