To solve this problem we will apply the concept related to kinetic energy based on the ideal gas constant and temperature. From there and with the given values we will find the temperature of the system. As the temperature is the same it will be possible to apply the root mean square speed formula that is dependent on the element's molar mass, the ideal gas constant and the temperature, this would be:
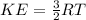
Where,
KE = Average kinetic energy of an ideal gas
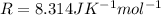 = Ideal gas constant
= Ideal gas constant
T = Temperature
Replacing we have,
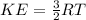
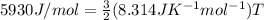
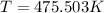
Therefore the temperature is 475.5K
RMS velocity of
 gas is
gas is
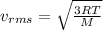
Where,
M = Molar mass of
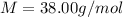
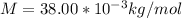
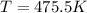
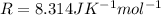
Replacing we have,
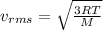
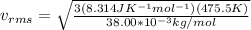
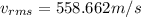
Therefore, the RMS velocity of
 gas is 558.6m/s
gas is 558.6m/s