Answer: The new penny is not pure copper
Step-by-step explanation:
Density
 is defined as a relation between the mass
is defined as a relation between the mass
 and the volume
and the volume
 :
:
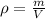
Now, we are told the density of pure copper is:
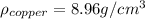
And we are given the mass and volume of the new penny, with which we can calculate its density:
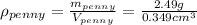
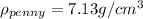 As we can see the density of this penny is not equal to the density of pure copper, hence the new penny is not pure copper.
As we can see the density of this penny is not equal to the density of pure copper, hence the new penny is not pure copper.