Answer:
The
 for the reaction
for the reaction
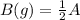 will be 4.69.
will be 4.69.
Step-by-step explanation:
The given equation is A(B) = 2B(g)
to evaluate equilibrium constant for
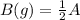
![K_c=[B]^2[A]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/nut2ohnupbetiihenmutqigzo0a0enwrnp.png)
= 0.045
The reverse will be
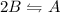
Then,
![K_c = ([A])/([B]^2)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/bdiu320bvkaw5yvaineyw57j2pn5l5xpm9.png)
=
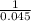
=
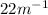
The equilibrium constant for
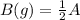 will be
will be
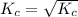
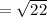
= 4.69
Therefore,
 for the reaction
for the reaction
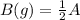 will be 4.69.
will be 4.69.