Answers:
a. Moles of oxygen formed: 0.670 mol
b. Moles of water formed: 1.34 mol
c. Mass of water formed: 24.1 g
d. Mass of oxygen formed: 21.4 g
Step-by-step explanation:
Dihdyrogen dioxide is the chemical name for a compound made of two hydrogen atoms and two oxide atoms, i.e. H₂O₂, which is also known as hydrogen peroxide or oxygenated water.
The decomposition reaction of dihydrogen dioxide into water and oxygen gas is represented by the balanced chemical equation:
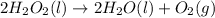
The mole ratios derived from that balanced chemical equation are:
- 2 mol H₂O₂ : 2 mol H₂O : 1 mol O₂
a. Moles of oxygen formed
- Set the proportion using the theoretical mole ratio of H₂O₂ to O₂ and the amount of moles of dyhydrogen dioxide that react:
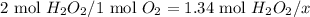
When you solve for x, you get:
- x = 1.34 mol H₂O₂ × 1 mol O₂ / 2 mol H₂O₂ = 0.670 mol O₂
b. Moles of water formed
- Set the proportion using the theoretical mole ratio of H₂O₂ to H₂O and the amount of moles of dyhydrogen dioxide that react:
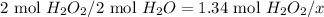
When you solve for x, you get:
- x = 1.34 mol H₂O₂ × 2 mol H₂O / 2 mol H₂O₂ = 1.34 mol H₂O
c. Mass of water formed
Using the number of moles of water calculated in the part b., you calculate the mass of water formed, in grams, using the molar mass of water:
- Molar mass of water = 18.015 g/mol
- Number of moles = mass in grams / molar mass
⇒ mass in grams = number of moles × molar mass
⇒ mass in grams = 1.34 mol × 18.015 g/mol = 24.1 g
d. Mass of oxygen formed
Using the number of moles of oxygen determined in the part a., you calculate the mass in grams using the molar mass of O₂.
- Molar mass of O₂ = 32.00 g/mol
- mass = molar mass × number of moles
- mass = 32.00 g/mol × 0.670 mol = 21.4 g.