Step-by-step explanation:
Given:
source temperature T1= 825° C = 1099 K
Sink temperature T2= 15°C = 288 K
Heat Supplied to the engine Qs= 3200 KW
a) Efficiency of a Carnot engine is
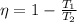

=0.7379
=73.79%
b)
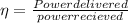
let W be the powe delivered
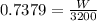
W= 2361.408 KW
c) Heat rejected to the cold temperature Qr= Qs-W
= 3200-2361.408=838.60 KW
d) Entropy change in the sink
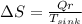
=838.60/288= 2.911 W/K