Answer:
The quantum number of the higher energy level is 7
Step-by-step explanation:
Given:
Momentum (p) = 0.3059×10⁻²⁷ kg·m/s
Planck's constant (h) = 6.626×10⁻³⁴ J·s
Speed of light (c) = 2.998×10⁸m/s
Charge of electron (e) = 1.602×10⁻¹⁹ C
Lower energy level (n₂) = 4
Higher energy level (n₁) = ?
From Bohr's model, change in energy of a photon is given as;
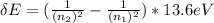
ΔE = P*C

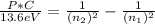
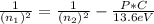
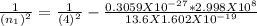
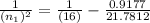
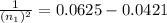
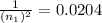
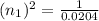
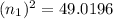
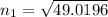
n₁ = 7
Therefore, the quantum number of the higher energy level is 7