Answer:

Step-by-step explanation:
We have given work is done on the gas is 1200 J
So work done will be
 ( as work is done on the gas )
( as work is done on the gas )
It is given that internal energy is increases by exactly 700 J
So
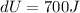
From thermodynamic equation
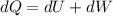
So
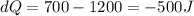
Here negative sign indicates that heat flow out of the gas
If heat was negative then heal was flowing in the gas