Answer: 0.05470atm
Step-by-step explanation:
The vapor pressure is determined by Clausius Clapeyron equation:
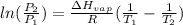
where,
 = initial pressure at = 1 atm (standard atmospheric pressure
= initial pressure at = 1 atm (standard atmospheric pressure
 = final pressure at
= final pressure at
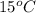 = ?
= ?
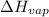 = enthalpy of vaporisation = 38.56 kJ/mol = 38560 J/mol
= enthalpy of vaporisation = 38.56 kJ/mol = 38560 J/mol
R = gas constant = 8.314 J/mole.K
 = initial temperature =
= initial temperature =
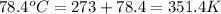
 = final temperature =
= final temperature =
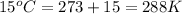
Now put all the given values in this formula, we get
![\log ((P_2)/(1atm))=(38560)/(2.303* 8.314J/mole.K)[(1)/(351.4K)-(1)/(288K)]](https://img.qammunity.org/2021/formulas/physics/high-school/oda2c7qanj5itgyd4n9jo3bbi3u3gcj0ku.png)
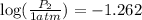
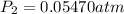
Thus the vapor pressure of ethanol at
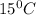 is 0.05470atm
is 0.05470atm