Answer:
Oxygen 35.6 percent
Carbon 60 percent
Hydrogen 4.4 percent
Step-by-step explanation:
Molecular mass of aspirin
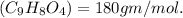
Mass of carbon in 180 gm of aspirine
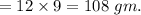
Therefore, percentage of carbon in aspirin is
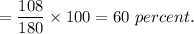
Similarly, mass of hydrogen in 8 gm of aspirine
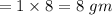 .
.
Hydrogen's percentage in aspirin
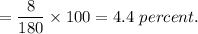
Also, mass of oxygen in aspirin is
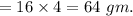
Oxygen's percentage in aspirin
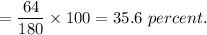
Hence, this is the required solution.