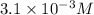Answer:
Solubility of nitrogen in water at a nitrogen pressure of 4.5 atm is
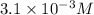
Step-by-step explanation:
According to Henry's law for solubility of a gas dissolved in a solvent-
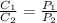
where
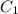 and
and
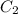 are solubility of the gas at a pressure of
are solubility of the gas at a pressure of
 and
and
 respectively.
respectively.
Here,
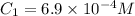 ,
,
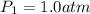 and
and
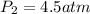
So,
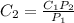
or,
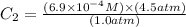
or,
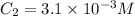
So, solubility of nitrogen in water at a nitrogen pressure of 4.5 atm is