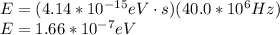Answer:
(a)
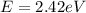
(b)
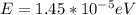
(c)
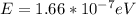
Step-by-step explanation:
The Planck-Einstein relation allows us to know the energy (E) of a photon, knowing its frequency (f). According to this relation, the energy of the photon is defined as:
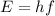
Here h is the Planck constant.
(a)
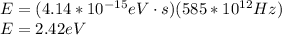
(b)
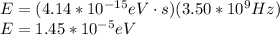
(c)