Answer:
Volume will increase by factor 2
So option (A) will be correct answer
Step-by-step explanation:
Let initially the volume is V pressure is P and temperature is T
According to ideal gas equation
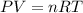 , here n is number of moles and R is gas constant
, here n is number of moles and R is gas constant
So
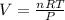 ....................eqn 1
....................eqn 1
Now pressure is doubled and temperature is quadrupled
So new volume
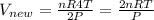 ........eqn 2
........eqn 2
Now comparing eqn 1 nad eqn 2
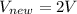
So volume will increase by factor 2
So option (A) will be correct answer