The question is incomplete, here is the complete question:
Consider the chemical reaction:
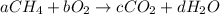
where a, b, c, and d are unknown positive integers. The reaction must be balanced; that is, the number of atoms of each element must be the same before and after the reaction. For example, because the number of oxygen atoms must remain the same, 2b=2c+d. While there are many possible choices for a, b, c, and d that balance the reaction, it is customary to use the smallest possible integers.
Balance this reaction.
Answer: The value of a, b, c and d in the given chemical equation are 1, 2, 1 and 2 respectively.
Step-by-step explanation:
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
This also means that total mass on the reactant side must be equal to the total mass on the product side.
For the given chemical reaction:
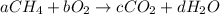
On reactant side:
Number of carbon atoms = 1
Number of hydrogen atoms = 4
Number of oxygen atoms = 2
On product side:
Number of carbon atoms = 1
Number of hydrogen atoms = 2
Number of oxygen atoms = 3
We need to balance the number of hydrogen and oxygen atoms. So, the balanced equation for the given reaction becomes:
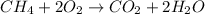
Hence, the value of a, b, c and d in the given chemical equation are 1, 2, 1 and 2 respectively.