Answer:
There are
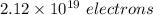 that must be removes from an electrically neutral silver dollar.
that must be removes from an electrically neutral silver dollar.
Step-by-step explanation:
In this case, we need to find the number of electrons must be removes from an electrically neutral silver dollar to give it a charge of +3.4 C. Let n number of electrons removed. It is a case of quantization of electric charge. It is given by :
q = ne
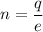
e is the charge on an electron
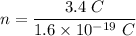
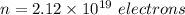
So, there are
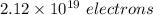 that must be removes from an electrically neutral silver dollar.
that must be removes from an electrically neutral silver dollar.