Answer: The vapor pressure of water at 298 K is 3.565kPa.
Step-by-step explanation:
The vapor pressure is determined by Clausius Clapeyron equation:
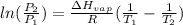
where,
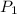 = initial pressure at 298 K = ?
= initial pressure at 298 K = ?
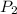 = final pressure at 373 K = 101.3 kPa
= final pressure at 373 K = 101.3 kPa
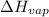 = enthalpy of vaporisation = 41.1 kJ/mol = 41100 J/mol
= enthalpy of vaporisation = 41.1 kJ/mol = 41100 J/mol
R = gas constant = 8.314 J/mole.K
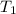 = initial temperature = 298 K
= initial temperature = 298 K
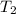 = final temperature = 373 K
= final temperature = 373 K
Now put all the given values in this formula, we get
![\log ((101.3)/(P_1))=(41100)/(2.303* 8.314J/mole.K)[(1)/(298K)-(1)/(373K)]](https://img.qammunity.org/2021/formulas/chemistry/college/u3qlbw5g84xnax1u44om0ztkwatjuyb47w.png)
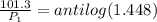
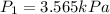
Therefore, the vapor pressure of water at 298 K is 3.565kPa.