Answer:
The answer to your question is 8.28 g of glucose
Step-by-step explanation:
Data
Glucose (C₆H₁₂O₆) = ?
Ethanol (CH₃CH₂OH)
Carbon dioxide (CO₂) = 2.25 l
Pressure = 1 atm
T = 295°K
Reaction
C₆H₁₂O₆ ⇒ 2C₂H₅OH(l) +2CO₂(g)
- Calculate the number of moles
PV = nRT
Solve for n
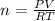
Substitution
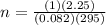
Simplification
n = 0.092
- Calculate the mass of glucose
1 mol of glucose --------------- 2 moles of carbon dioxide
x --------------- 0.092 moles
x = (0.092 x 1) / 2
x = 0.046 moles of glucose
Molecular weight of glucose = 180 g
180 g of glucose --------------- 1 mol
x g ---------------0.046 moles
x = (0.046 x 180) / 1
x = 8.28 g of glucose