Answer:
Concentration of
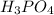 in sample is 0.25 M.
in sample is 0.25 M.
Step-by-step explanation:
From the reaction, one mole of
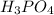 reacts with 3 moles of NaOH.
reacts with 3 moles of NaOH.
Now, number of moles of NaOH, n =
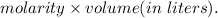
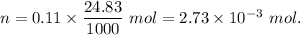
Therefore,
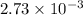 mol of NaOH reacts with
mol of NaOH reacts with
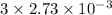
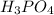 .
.
So, concentration of
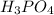
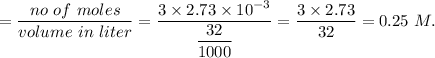
Therefore, concentration of
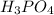 in sample is 0.25 M.
in sample is 0.25 M.
Hence, this is the required solution.