Answer:
Lowering the temperature typically reduces the significance of the decrease in entropy. That makes the Gibbs Free energy of the reaction more negative. As a result, the reaction becomes more favorable overall.
Step-by-step explanation:
In an addition reaction there's a decrease in the number of particles. Consider the hydrogenation of ethene as an example.
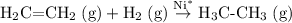 .
.
When
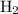 is added to
is added to
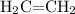 (ethene) under heat and with the presence of a catalyst,
(ethene) under heat and with the presence of a catalyst,
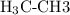 (ethane) would be produced.
(ethane) would be produced.
Note that on the left-hand side of the equation, there are two gaseous molecules. However, on the right-hand side there's only one gaseous molecule. That's a significant decrease in entropy. In other words,
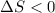 .
.
The equation for the change in Gibbs Free Energy for a particular reaction is:
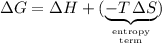 .
.
For a particular reaction, the more negative
 is, the more spontaneous ("favorable") the reaction would be.
is, the more spontaneous ("favorable") the reaction would be.
Since typically
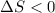 for addition reactions, the "entropy term" of it would be positive. That's not very helpful if the reaction needs to be favorable.
for addition reactions, the "entropy term" of it would be positive. That's not very helpful if the reaction needs to be favorable.
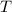 (absolute temperature) is always nonnegative. However, lowering the temperature could help bring the value of
(absolute temperature) is always nonnegative. However, lowering the temperature could help bring the value of